UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
(Exact name of Registrant as Specified in Its Charter)
| (State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
| |
||||
| (Address of Principal Executive Offices) | (Zip Code) |
(Registrant’s Telephone Number, Including Area Code)
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instructions A.2. below):
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered | ||
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
| ITEM 8.01 | OTHER EVENTS |
RAPT Therapeutics, Inc. (the “Company”) is filing the investor presentation slides (the “Corporate Presentation”) attached as Exhibit 99.1 to this Current Report on Form 8-K, which the Company may use from time to time in conversations with investors and analysts.
| ITEM 9.01 | FINANCIAL STATEMENTS AND EXHIBITS |
(d) Exhibits
| Exhibit Number |
Exhibit Description | |
| 99.1 | Corporate Presentation | |
| Exhibit 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document). | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| RAPT Therapeutics, Inc. | ||||||
| Dated: September 27, 2021 | By: | /s/ Rodney Young | ||||
| Rodney Young | ||||||
| Chief Financial Officer | ||||||

Transforming the Treatment of Cancer and Inflammation Corporate Presentation September 2021 Exhibit 99.1

Legal Disclaimers Statements in this Presentation that are not statements of historical fact are forward-looking statements. Such forward-looking statements include, without limitation, statements regarding RAPT Therapeutics, Inc.’s (the "Company," "we," or "us") research and clinical development plans; current and future drug candidates; business strategy and plans; regulatory pathways; and our ability to complete certain milestones. Words such as "believe," "anticipate," "plan," "expect," "will," "may," "upcoming," "milestone," "potential," "target" or the negative of these terms or similar expressions are intended to identify forward-looking statements, though not all forward-looking statements necessarily contain these identifying words. These forward-looking statements are based on the current beliefs of the Company's management with respect to future events and trends and are subject to known and unknown risks and uncertainties, including those described in the “Risk Factors” section of our most recent Form 10-Q filed with the Securities and Exchange Commission, and any current and periodic reports filed thereafter, that may cause our actual performance or achievements to be materially different from any future performance or achievements expressed or implied by the forward-looking statements in this Presentation. These forward-looking statements should not be taken as forecasts or promises nor should they be taken as implying any indication, assurance or guarantee that any assumptions on which such forward-looking statements have been made are correct or exhaustive or, in the case of such assumptions, fully stated in the Presentation. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date this Presentation is given. Although we believe that the beliefs and assumptions reflected in the forward-looking statements are reasonable, we cannot guarantee future performance or achievements. Except as required by law, we undertake no obligation to update publicly any forward-looking statements for any reason after the date of this Presentation. This Presentation discusses drug candidates that are under clinical study and which have not yet been approved for marketing by the U.S. Food and Drug Administration. No representation is made as to the safety or effectiveness of any drug candidates for any use for which such drug candidates are being studied.

DISCOVERY Oral Drugs Targeting Critical Immune Drivers of Disease RPT193 (Inflammation): Oral agent targets inflammatory Th2 cells PoC in Phase 1b achieved in AD: efficacy on all key exploratory endpoints with excellent safety and tolerability FLX475 (Oncology): Selectively targets immunosuppressive tumor Treg PoC in Phase 2 with monotherapy and combo activity observed HPK1 (Oncology) Other Targets CLINICAL Proprietary discovery engine Diversified pipeline Large market opportunities Multiple near-term clinical readouts Strategic collaborations

Proprietary Drug Discovery and Development Engine R Drug discovery Clinical development to POC Rapid Interrogating clinically-relevant big datasets to identify targets and biomarkers Driven by data to improve chances of clinical success Critical immune drivers of cancer and inflammation A Analytics P Patient selection T Targeting

RPT193: CCR4 Antagonist for Inflammatory Diseases

RPT193: Oral CCR4 Antagonist for Inflammatory Diseases RPT193 is a highly potent and selective once-daily oral CCR4 antagonist that targets inflammation more specifically than JAK inhibitors and acts upstream of the injectables Phase 1b trial demonstrated clear benefit in patients with moderate-to-severe AD, with favorable safety and tolerability No laboratory safety monitoring or black box warning expected Next steps: Phase 2b trial in AD and a Phase 2a trial in asthma Normal Human Skin AD Lesional Skin

RPT193 Targets Th2 Cells: Key Drivers of Inflammation in Atopic Dermatitis, Asthma, and Other Diseases Epithelial Barrier Surface CCR4 Th2 IL-5 IL-4 IL-13 Inflammation Thickening Itch Signaling via CCR4 regulates Th2 cell migration into inflamed tissues and enhances cytokine secretion Cytokines Allergen, Microbes RPT193 is a potent and selective oral CCR4 antagonist that specifically inhibits Th2 cell migration, function and activation. CCL17 CCL22 anti-IL4Rα Ab anti-IL5/R Ab anti-IL13 Ab

Atopic Dermatitis and Asthma Represent Major Markets Atopic Dermatitis (AD) Common disease affecting ~19M adults and ~10M children in the US $24B projected market by 2029* Asthma Asthma affects ~15M adults and children in the US $21B projected market by 2029* High unmet need: a well-tolerated, safe and effective, oral drug that does not require laboratory safety monitoring RPT193 has the potential to address this unmet need * Decision Resources Guide; EU, US, and Japan market

Enrolled 31 patients into a double-blind, randomized trial with 2:1 allocation of RPT193 to placebo Monotherapy study: steroid and immunosuppressant washout period; rescue steroids not permitted through Day 43 Trial was not powered for any specific endpoint Exploratory endpoints include: EASI, Pruritis Numerical Rating Scale (NRS), and vIGA Data presented are from the Intent to Treat dataset Phase 1b Trial Explored RPT193 Activity in Patients with Moderate-to-Severe Atopic Dermatitis Obtain Informed Consent ≥12-month history of AD 18-65 years of age (inclusive) BMI ≥18 and <40 kg/m2 BSA ≥10% EASI ≥12 vIGA ≥3 Screening (Up to 35 days) Day -35 -1 RPT193 400 mg once daily Placebo Randomization (2:1) Treatment (28 days) Follow-up (14 days) 1 29 8 15 43 Study Assessments (Day 1 to 43)

Phase 1b Baseline Demographics Placebo RPT193 N 10 21 Age, Mean (Range) 35.8 (22-64) 41.0 (19-63) Female, n (%) 4 (40.0%) 12 (57.1%) Baseline Characteristics EASI, Mean (Range) 21.07 (13.6-45.5) 18.49 (12-30) BSA, Mean (Range) 24.5 (10-61) 23.3 (11-55) vIGA 3, n (%) 8 (80.0%) 18 (85.7%) Peak NRS, Mean (Range) 7.3 (3-10) 6.9 (3-10) Peak NRS ≥4, n (%) 9 (90.0%) 20 (95.2%)

RPT193 Differentiated from Placebo for EASI and EASI-50 at Day 29 with Further Improvement at Day 43 % Improvement in EASI Proportion of EASI-50 19.3% 43.6% 32.9% 41.9% Follow-up Treatment Follow-up Treatment *p < 0.05 * *
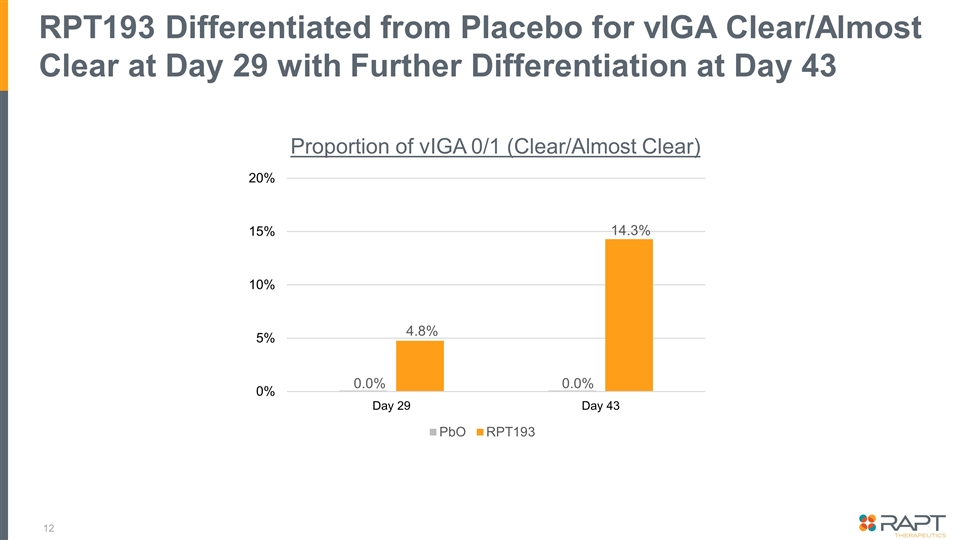
RPT193 Differentiated from Placebo for vIGA Clear/Almost Clear at Day 29 with Further Differentiation at Day 43 0.0% 0.0% 4.8% 14.3% Proportion of vIGA 0/1 (Clear/Almost Clear)

RPT193 Demonstrated Clinically Meaningful Improvement in Itch Compared to Placebo at Day 29 Proportion of NRS-4* 22.2% 45.0% *At least a 4-point improvement among patients with a baseline pruritis NRS ≥4

Phase 1b Safety No SAEs reported All AEs reported were mild or moderate in intensity No clinically significant safety laboratory abnormalities observed Overall safety profile to date suggests a well-tolerated oral drug that should not require laboratory safety monitoring

RPT193 6-Week Efficacy Compared to Other Drugs at 12-16 Weeks* RPT193 RPT193 400 mg Biologics Orals Dupilumab Ph3 (300 mg q2wk) Lebrikizumab Ph2 (250 mg q2wk) Tralokinumab Ph3 (300 mg q2wk) Abrocitinib Ph3 (200 mg) Baricitinib Ph3 (2 mg) Etrasimod Ph2 (2 mg) Upadicitinib Ph2,3 (15 mg) % EASI improvement EASI-50 vIGA 0 or 1 100 RPT193 RPT193 0 40 20 60 All data shown are placebo-adjusted * Comparisons are based on published data and relative properties of other agents and do not reflect a head-to-head comparative study or clinical trial

Next Steps for the RPT193 Program RPT193 Phase 2 Trials Phase 1b Atopic Dermatitis Phase 2b Atopic Dermatitis Phase 2a Asthma PoC Achieved Adults with moderate-to-severe AD 16-week dosing period Multiple dose levels of RPT193 vs. placebo Goal: Evaluate efficacy and safety at 16 weeks and determine optimal dose for Phase 3 trials In planning

RPT193 Program Summary Data from the Phase 1b study in patients with atopic dermatitis demonstrated clear benefit on all key exploratory clinical endpoints including EASI and vIGA Continued deepening of responses through the 2-week follow-up period suggests higher levels of efficacy could be achieved in longer studies Profile suggests an effective, well-tolerated oral molecule not needing laboratory safety monitoring, with positioning ahead of injectables and JAK inhibitors Additional late-breaking Phase 1b data to be presented at EADV Congress Next steps: 16-week Phase 2b study in patients with moderate-to-severe AD and a Phase 2a study in patients with asthma

FLX475: CCR4 Antagonist for Oncology

Treg Are Key Targets in the Tumor Microenvironment (TME) Correlate with poor prognosis across most cancers Mechanism for immune evasion by viruses and tumors Barrier to checkpoint inhibitor efficacy Challenge: selective inhibition of Treg in the TME Depleting antibodies targeting CD25, CCR4, etc. do not appear to have adequate selectivity Treg CD8 Cancer Types Bruni D et al. Nat Rev Cancer 2020

FLX475: Oral CCR4 Antagonist in Phase 2 Highly potent and selective CCR4 small molecule antagonist Selectively blocks tumor Treg while sparing normal tissues and beneficial cells Potential for superior safety and efficacy compared to depleting antibodies Issued U.S. composition of matter patent with coverage through 2037 Monotherapy and combination antitumor activity in charged cancers Blocks interaction with CCR4 ligands CCL22 and CCL17 on Treg

Identification and Characterization of Charged Tumors “Charged” tumors: high levels of CCR4 ligands, Treg and CD8 T cells Potential for both monotherapy and combination activity Represent cancers with high unmet need and large markets Potential for tissue-agnostic accelerated approval in virally- associated tumors Data from in-house analysis of TCGA database combined with other data sets; Confirmed in > 400 tumor microarrays The graph above reflects a logarithmic scale on each axis NPC Nasopharyngeal; HNSCC Head & Neck Squamous Cell Carcinoma; NHL Non-Hodgkin Lymphoma; NSCLC Non-Small Cell Lung Cancer; TNBC Triple Negative Breast Cancer Treg CCR4 Ligands CD8 Signature Gastric EBV+ NPC EBV+ NSCLC Sq. TNBC Cervical HPV+ HNSCC NSCLC Ad. Virally-Associated Non-Virally-Associated NHL

Phase 2: Gated Simon 2-Stage Design FLX475 Monotherapy FLX475 w/ Pembrolizumab Nasopharyngeal (EBV+) Non-Small Cell Lung (CPI Experienced) Hodgkin/Non-Hodgkin Lymphoma (EBV+) Head & Neck (CPI Naïve) Cervical (HPV+) (CPI Naïve) TN Breast (CPI Naïve) Head & Neck (CPI Naïve) Head & Neck (CPI Experienced) Stage 2 N=19 per cohort Stage 1 N=10 per cohort Endpoints: Safety, PK, Biomarkers, Tumor Response Assessment CPI = Checkpoint Inhibitor To evaluate the antitumor activity of FLX475 as monotherapy and in combination with pembrolizumab in charged cancers that progressed after ≥ 1 line of therapy

Updated Stage 2 Decisions (September 2021) Nasopharyngeal (EBV+) Monotherapy Nasopharyngeal (EBV+) Monotherapy Nasopharyngeal (EBV+) Combination Go to Stage 2 Stop Stage 1 Stage 2 Decision EBV+ Lymphoma Monotherapy EBV+ Lymphoma Mono and Combo Stage 1 Pending NSCLC (CPI Experienced) Combination Cervical (CPI Naïve) Monotherapy TN Breast (CPI Naïve) Combination Head & Neck (CPI Exp.) Combination Head & Neck (CPI Naïve) Monotherapy Head & Neck (CPI Naïve) Monotherapy Head & Neck (CPI Naïve) Combination Head & Neck (CPI Exp.) Combination NSCLC (CPI Experienced) Combination TN Breast (CPI Naïve) Combination Head & Neck (CPI Naïve) Combination

FLX475 Phase 2 Program Summary FLX475, a highly selective tumor Treg inhibitor, appears to be an active agent in charged cancers Demonstrated clinical activity as monotherapy Demonstrated clinical activity in combination with pembrolizumab in checkpoint-naïve cancers beyond expected from checkpoint alone Ungated Stage 2 expansions in 4 indications EBV+ lymphoma, nasopharyngeal and head and neck cancers (CPI naïve and CPI refractory) Favorable safety supportive of broad combinability Targeting a medical conference in 2022 for data presentation

Key Takeaways and Upcoming Milestones RPT193: safe oral agent designed for an array of inflammatory diseases – Positive Phase 1b data in AD FLX475: a highly selective tumor Treg inhibitor in multiple Phase 2 expansions as monotherapy and in combination with pembrolizumab Next Key Milestones Sept 2021: RPT193 late-breaking presentation at EADV Congress 2021 1H 2022: RPT193 Phase 2b AD trial initiation 2022: FLX475 Phase 2 data update 2022: RPT193 Phase 2a asthma trial initiation

Thank You
